Research Area
Bioorganic Chemistry, Medicinal Chemistry, Chemical Biology, Biophysics
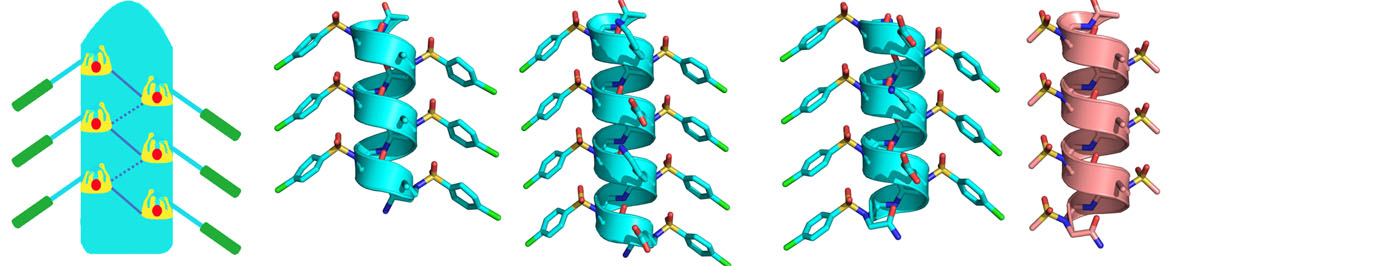
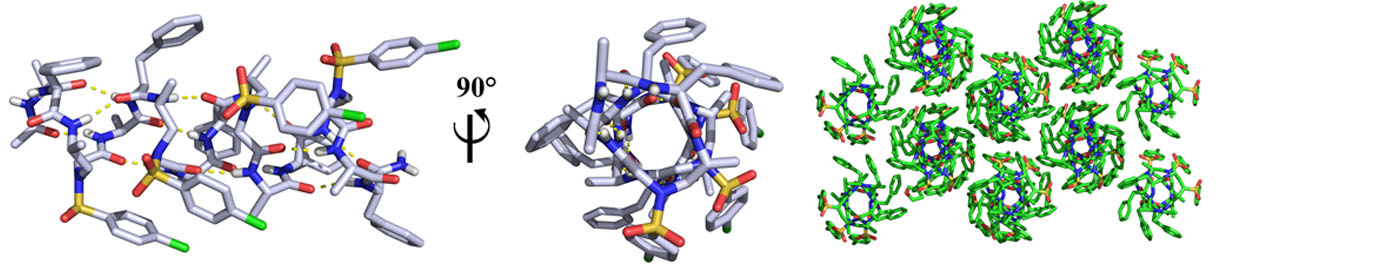
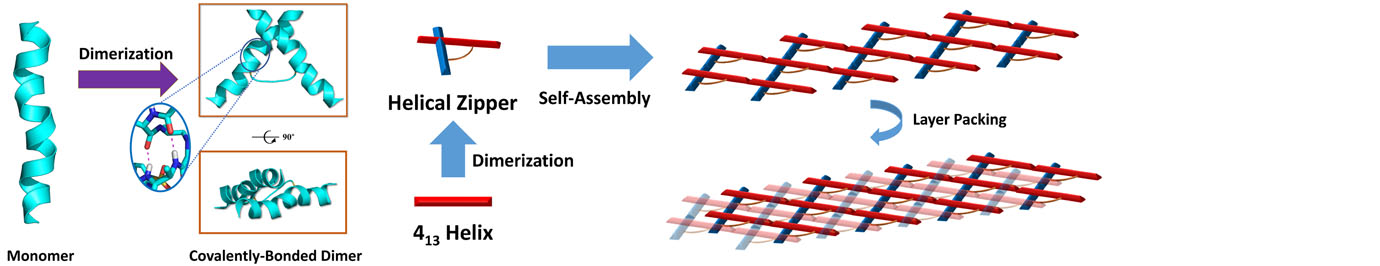
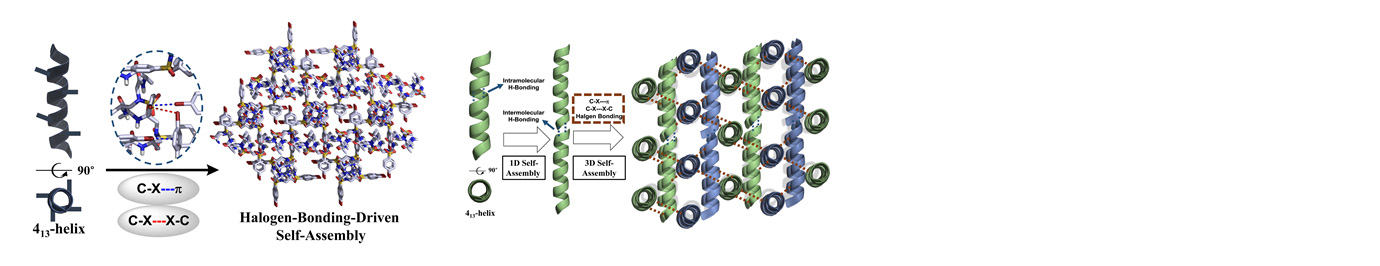
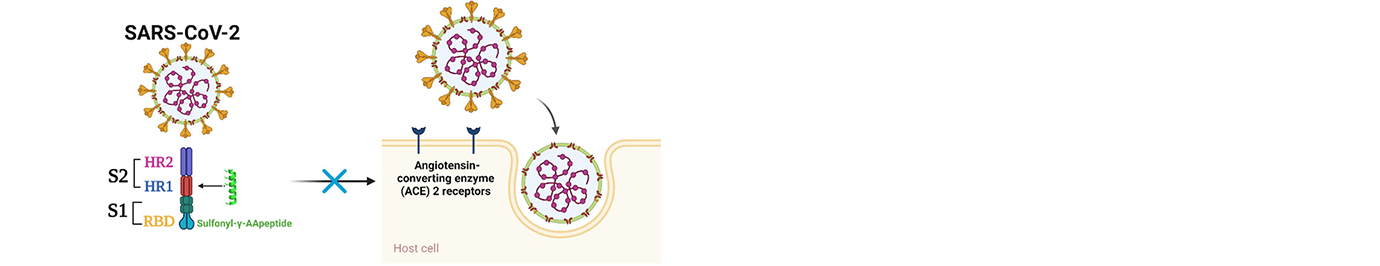
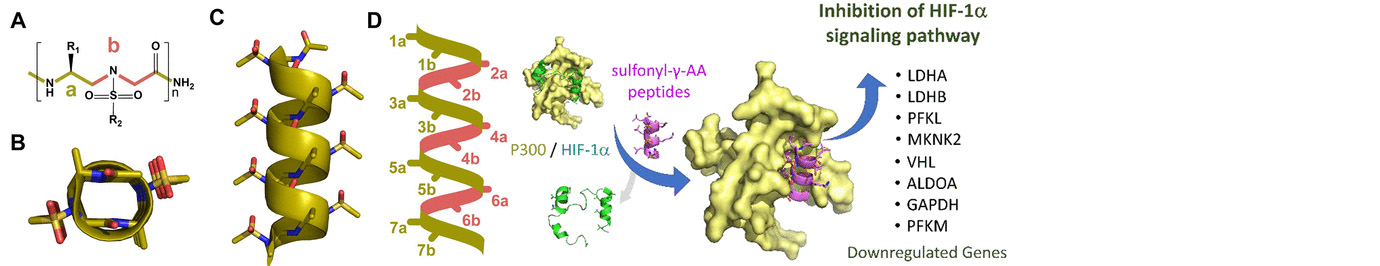
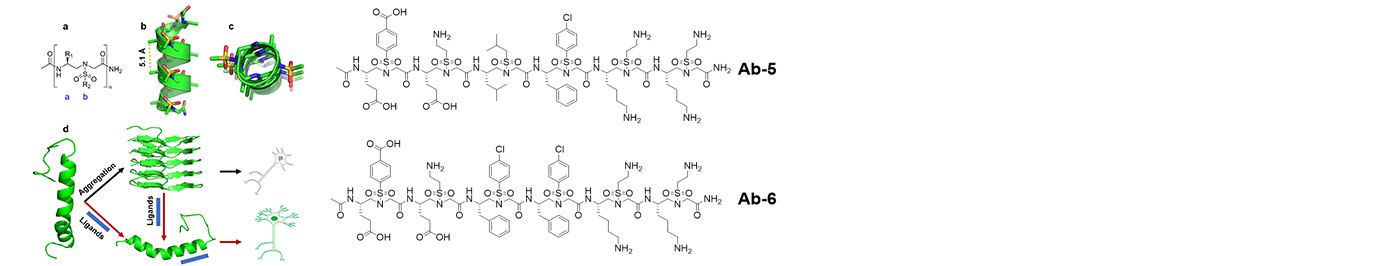
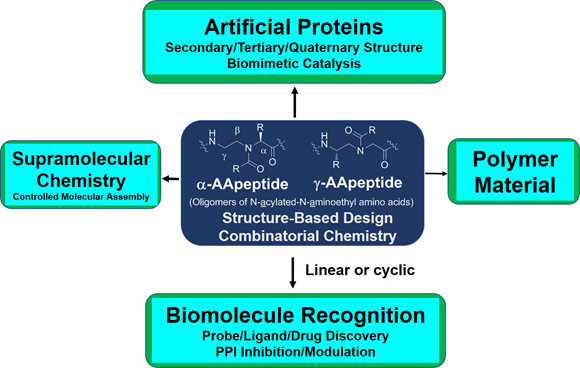
The research in our laboratory is focused on the expansion of interdisciplinary research at the interface of chemistry and biology to design and synthesize novel peptidomimetics and small molecules that modulate protein-protein interactions involved in critical cellular processes. Understanding how these compounds interact with proteins will help to reveal mechanisms governing cell activities. We are particularly interested in developing compounds that lead to the design of novel therapeutics potentially targeting cancer, HIV, and Alzheimer's disease, etc. Specifically, we have developed a new class of sequence-specific peptidomimetics-AApeptides, inspired by chiral PNA backbones. Our research findings reveal that AApeptides could fold into well-defined protein-like secondary and tertiary structures, and they display remarkable biological potential for the recognition of protein and nucleic acids. As such, AApeptides could be developed into a valuable tool and drug candidates that probe or modulate medicinally relevant cellular processes. We thus employ two complementary approaches: structure-based design and combinatorial screening to design and identify AApeptides that disrupt disease-related protein-protein interactions and cell signaling. In addition to translational research related to medicinal chemistry/drug discovery, we also have keen interest in fundamental science. Based on AApeptide scaffold, we hope to develop new artificial proteins including enzymes with distinct and predictable functions. In the meantime, we also explore their application in supramolecular chemistry and biomaterials through controlled self-assembly. The scheme below sketches our long-term effort. A few research directions are also highlighted here. Please refer to our research page for more detailed information.
Research Focus
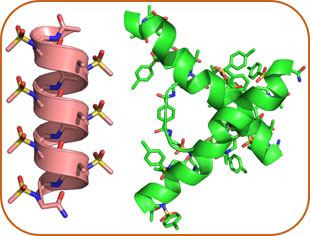
AApeptide Folding
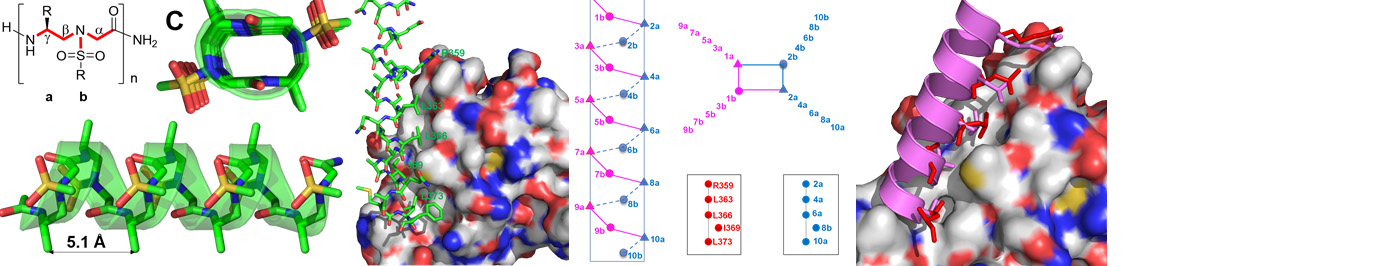
Exloration of secondary, tertiary and quaternary of AApeptides, based on which functions such as enzymatic activity and organic catalysis are investigated.
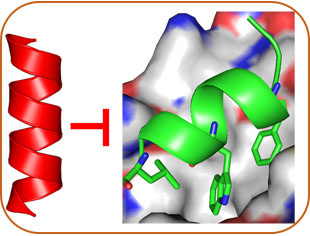
Structure-Based Design
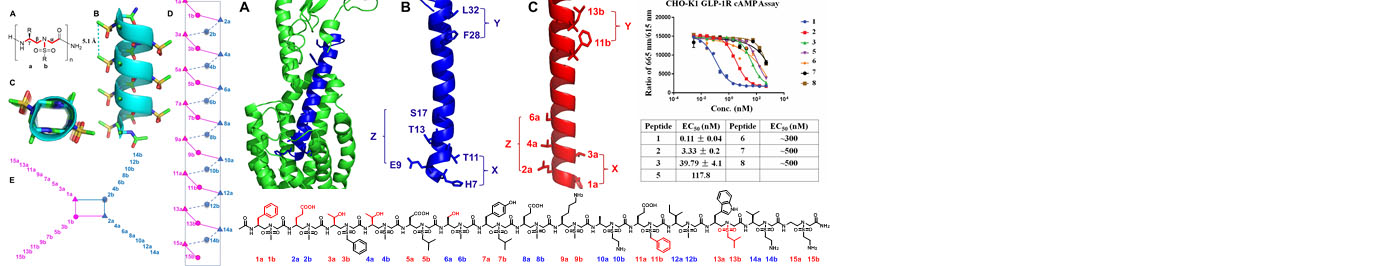
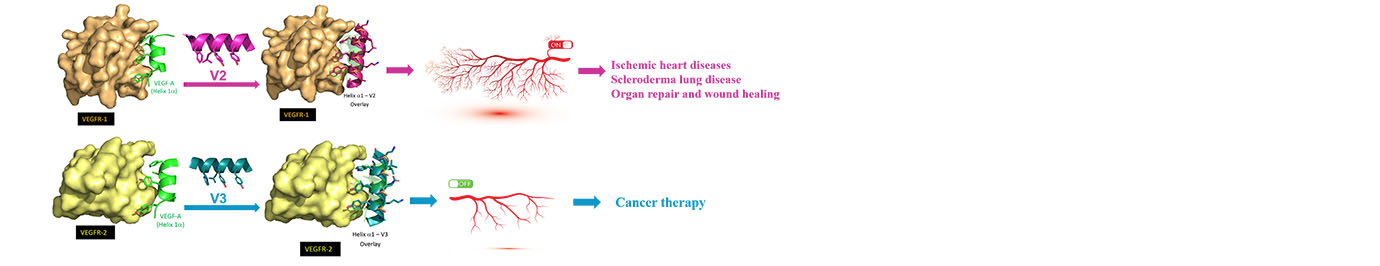
Based on the helical structures of AApeptides, inhibitors are designed to disrupt disease-related protein-protein interactions such as cancer, HIV, diabetes.
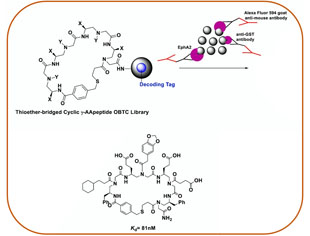
Combinatorial Screening
Making use of endless chemodiversity, AApeptide combinatorial library screening provides alternative approach to identify molecular probes and drug candidates.
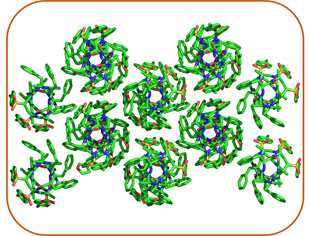
Supramolecular Chemistry
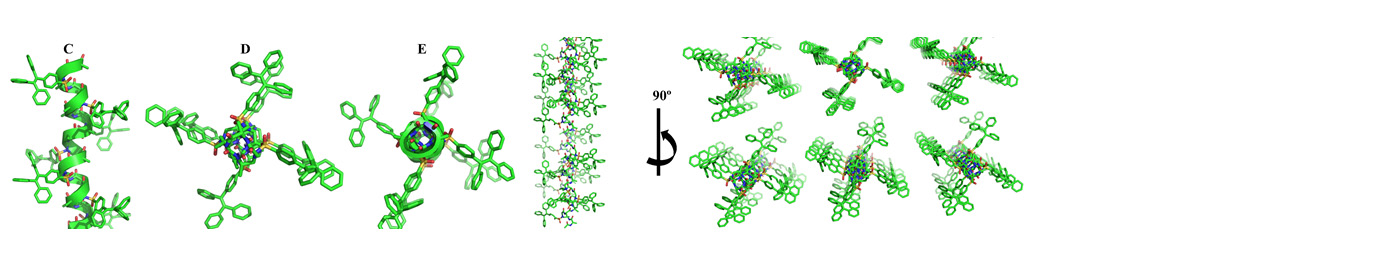
Precise manipulation of functional groups on AApeptides lead to controlled self-assembly. The richness in supramolecular chemistry could lead to various functional materials.